As you may well know, metals come in all kinds of shapes and sizes. Some can be bended at will and some are sturdy as a rock (pun intended).
Ultimately when you go down at the atomic level and look at these same metals closely, the difference in structure is almost negligible.
We use what is known as a unit cell to differentiate these metals apart. By merely looking at the unit cell of a material, you can already grasp the larger structure and even properties of that material. How is that even possible?
Well, in this article, we shall take you on a journey to fully understand how the unit cell shapes the crystal structure of metals. By the end of this journey, you should be able to upon sight – tell what type of crystal structure a material is and even list some properties that it will have.
Crystal structures in Metals
Metals are made up of repeating arrangements of atoms which form a crystal structure.
This crystal structure can be imagined as a three dimensional pattern in which atoms are arranged layer by layer in all directions. Depending on how these atoms are arranged, properties like density, hardness, and electrical conductivity can be determined.
Typically, any crystal structure can be defined in terms of it’s unit cell.
Because the unit cell is the smallest unit, when repeated and stacked in three dimensions—will create the entire lattice of a crystal structure with subatomic accuracy.
That is why grasping the unit cell is all you will need when trying to understand the larger structure.
What exactly is the Unit Cell?
Now we’ve referenced the unit cell constantly, and you might even begin to have a fuzzy impression of what it might be, but what exactly is unit cell?
The unit cell is an imaginary box that contains a set of atoms arranged in a particular manner. When the atoms are repeated in 3-dimensional space, the entire crystal structure is created. When defining a unit cell or even crystal structures in general, the following parameters need to be known:
- Lattice Parameters/ Lattice Constant: These parameters give an accurate measurement of the geometry of the unit cell. They include the lengths of the edges of the unit cell (a, b, c) and each of the angles between them (α, β, γ).
- Symmetry: This is the way that the unit cell can be reflected or rotated without a change in its overall appearance.
- Coordination Number: This is the number of neighboring or more precisely, touching atoms to a single atom.
- Atomic Packing Factor (APF): This is the ratio of the volume of atoms that can be contained in a unit cell. APF is calculated by dividing the volume of atoms in a unit cell by the total unit cell volume.
\[\text{APF} = \frac{\text{volume of atoms in a unit cell}}{\text{total unit cell volume}}\]
As we move through the different unit cells, we will be calling back to the terms above. It is essential to know what they mean.
Types of Crystal structures in Metals
When it comes to the classification of crystal structures, there are a total of 7 unique Lattice systems with a total of 14 different Bravais Lattices. When it comes to metals, the most common types of crystal structures are the following:
- Simple Cubic (SC)
- Body-Centered Cubic (BCC)
- Face-Centered Cubic (FCC)
- Hexagonal Close-Packed (HCP)
The reason that most metals fall under the structures above is mostly due to their efficiency of APF.
Simple Cubic (SC) crystal structure
The most basic crystal structure is the simple cubic crystal structure.
Each section of atom in a SC is positioned at a corner of its unit cell. SC has 1 atom per unit cell. It has a coordination number of 6 atoms, meaning that a total of 6 atoms are always touching a single atom. The lattice constant of SC can be defined by \(a=2r\). This means that each side of the cube is the same as the radius of the atom multiplied by 2. The atomic packing factor of SC is 0.52 or about 52%. This means that the volume of atom that is contained in the unit cell only covers about 52% of the total volume of the unit cell.
Below is a diagram of a SC crystal structure:
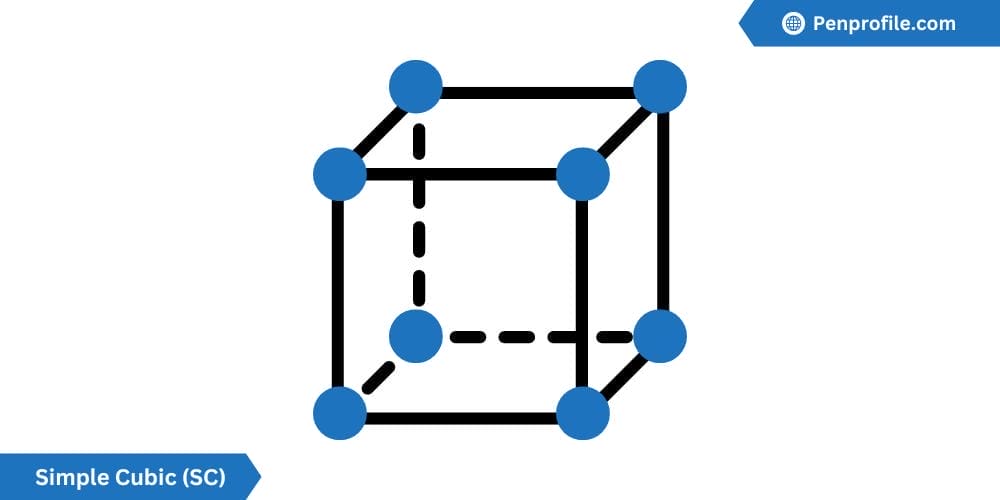
The simple cubic crystal structure is relatively rare. So much so that only 1 known element at a constant temperature and pressure has this structure. Polonium (Po) is said element. The reason for its rarity is the low APF of 52% making it very unstable, in fact Polonium is an extremely radioactive element.
Elements with the SC crystal structure tend to have the following properties:
- Low density
- Low shear strength
- Brittleness
- Lower elastic modulus
To recap, SC has:
Atom per unit cell: 1
Coordination number: 6
Lattice constant: \(a=2r\).
APF: 0.52 / 52%
Body-Centered Cubic (BCC) crystal structure
In the simplest way to understand, a body-centered cubic structure (BCC) is just a SC with an additional atom smack dap in the middle surrounded by the corner pieces.
The BCC has 2 atoms per unit cell. It has a coordination number of 8 atoms, each touching one another with a lattice constant given by \(a = \frac{4R}{\sqrt{3}}\) .The BCC also has an atomic packing factor of 0.68 meaning that about 68% of its volume is occupied by atoms.
Below is a diagram of a BCC crystal structure:
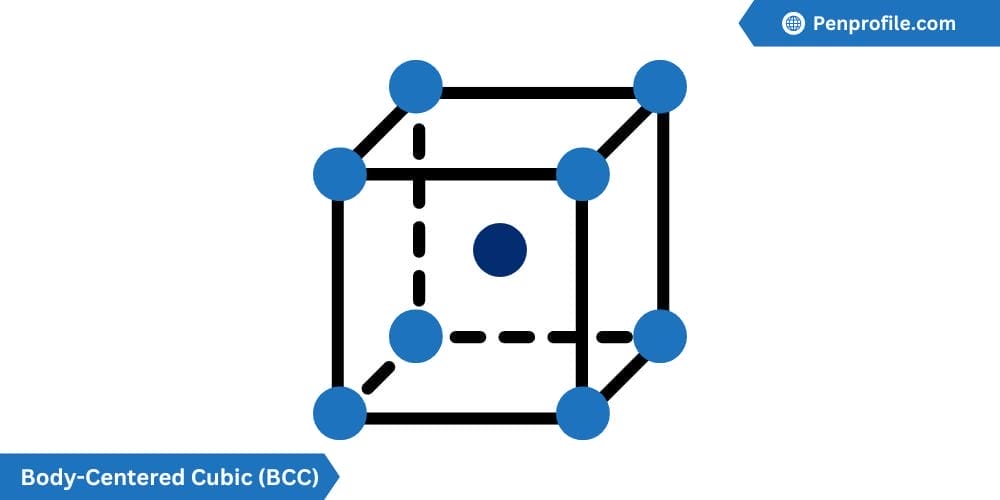
Due to its relatively high APF, the BCC can be found in many elements. Chromium (Cr), Tungsten (W), and Iron (Fe)-at room temperature are all examples of the BCC crystal structure
Elements with the BCC crystal structure tend to have the following properties:
- High yield strength
- High young modulus
- High hardness
- High tensile strength
To recap, the BCC crystal structure has:
Atom per unit cell: 2
Coordination number: 8
Lattice constant: \(a = \frac{4R}{\sqrt{3}}\)
APF: 0.68 / 68%
Face-Centered Cubic (FCC) crystal structure
The face-centered cubic (FCC) is one of the most efficient structures, as result many elements adopt it.
The FCC — just like the last two structures—has one-eighth of an atom at each of its corners. It also has one-half of an atom on all its faces, all combining to form a total of 4 atoms per unit cell. The FCC has a coordination number of 12 atoms with a lattice constant given by \(a=2R\sqrt{2}\). It has an atomic packing factor of a whopping 0.74 or about 74% of the volume of the unit cell. FCC is by far one of the most efficient structures available.
Below is a diagram of a FCC crystal structure:

The FCC structure can be found in many well-known elements such as Gold (Au), Silver (Ag), Copper (Cu), and Aluminum (Al) to name but a few.
Elements with the FCC crystal structure tend to have the following properties:
- Low yield strength
- Low hardness
- Low young modulus
- Good ductility
To recap, the FCC crystal structure has:
Atom per unit cell: 4
Coordination number: 12
Lattice constant: \(a=2R\sqrt{2}\)
APF: 0.74 / 74%
Hexagonal Closed-Packed (HCP) crystal structure
The hexagonal closed-packed (HCP) crystal structure is another highly efficient packing factor, consisting of layer upon layers of atoms all arranged in a hexagonal pattern.
The HCP contains 6 atoms per unit cell: one-sixth of each of the top and bottom face corner atoms, one-half of each of the center face atoms, and all three midplane interior atoms. The coordination number as well as the atomic packing factor for the HCP is the same as the FCC, so 12 and 0.74 or 74% respectively. The lattice constant for HCP is given by \(a=2R\).
Below is a diagram of a HCP crystal structure:
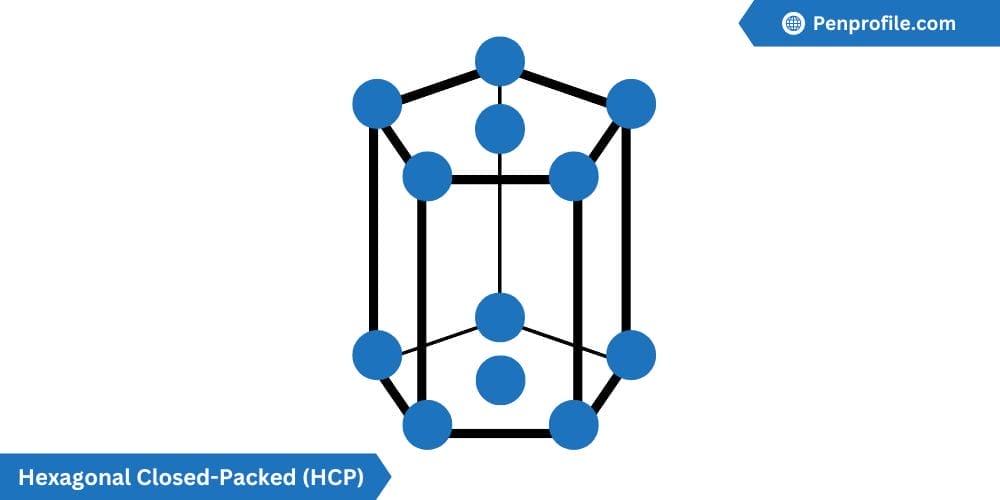
Titanium (Ti), Zinc (Zn), and Magnesium (Mg) are all examples of a HCP crystal structure.
Elements with the HCP crystal structure tend to have the following properties:
- Brittleness
- Low yield strength
To recap, the HCP crystal structure has:
Atom per unit cell: 6
Coordination number: 12
Lattice constant: \(a=2R\)
APF: 0.74 / 74%
Example problems
Now that we have understood what the unit cell is and the different types crystal structures for metals, let’s solve a few problems.
Problem 1
The atomic radius of an element with a BCC structure is 0.124nm. Calculate the atomic packing factor (APF) for this structure.
Solution:
Givens:
Atomic radius: r = 0.124nm.
- Calculate the lattice parameter (a) using the formula:
\(a=\frac{4r}{\sqrt{3}}\) - Substitute the given values into the formula:
\(a=\frac{4\cdot 0.124nm}{\sqrt{3}}=0.286nm\) - Calculate the volume of the unite cell:
\(V=a^3=(0.286nm)^3=0.0234nm^3\) - We know that for a BCC structure, 2 atoms are available.
- We then calculate the volume of 1 atom which is presumed to be a sphere, so:
\(V=\frac{4}{3}\pi r^3=\frac{4}{3}\pi(0.124)^3=0.008nm^3\) - So then the volume of 2 atoms will then be:
\(V= 2\cdot 0.008nm^3=0.0160nm^3\) - And now, we can simply use the APF formula to solve the problem:
\(APF=\frac{0.0160nm^3}{0.234nm^3}=0.68\)
Problem 2
Calculate the volume of a unit cel for an FCC crystal if the atomic radius of the metal is 0.137nm.
Solution:
Givens:
Atomic radius: 0.137nm
- First we calculate the lattice parameter (a) using the formula:
\(a=2\sqrt{2r}\) - Plug in the values
\(a=2\sqrt{2}\cdot 0.137nm=0.387nm\) - The volume of the unit cell can be determined by the following:
\(V=a^3=(0.387nm)^3 =0.05nm^3\)
In conclusion
By understanding the unit cell and the various types of crystal structures, one can unlock great potential in the field of material science. These concepts are fundamental in understanding how various materials and elements are structured. You will understand that just because these materials are visibility dissimilar, when looking at them through the lens of a unit cell, one can truly see how interconnect they are.
As promised, you should now be able to upon sight – tell what type of crystal structure a material is as well as even list some properties that it will have.















